Vivet’s lead program, VTX-801 for Wilson Disease, highlighted during Pfizer’s Investor Day September 15th 2020
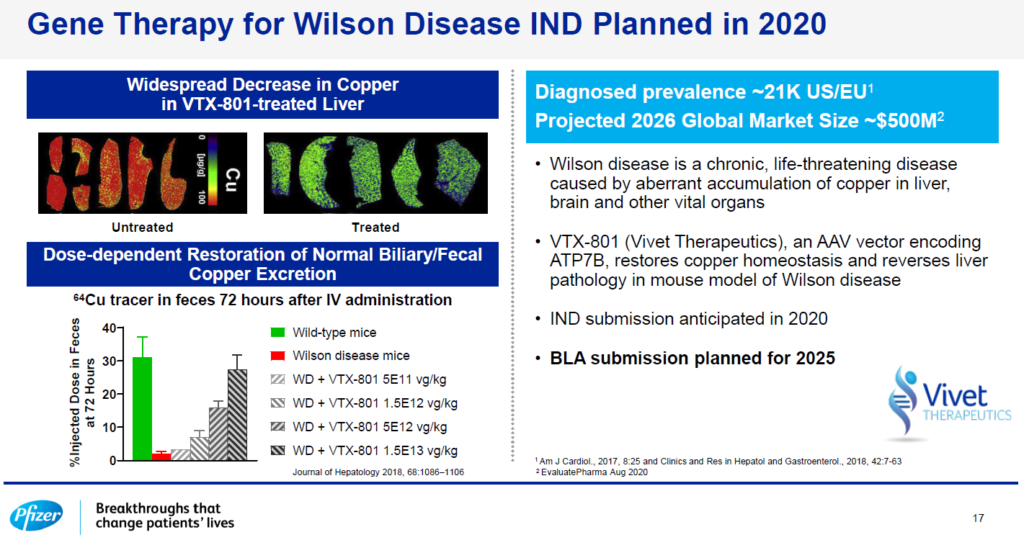
Vivet Therapeutics’ lead program, VTX-801 for Wilson Disease, was highlighted during Pfizer’s Investor Day September 15th 2020.
Vivet’s Second Gene Therapy Product, VTX-803 for PFIC3, Receives US and European Orphan Drug Designation.

PARIS, France June 1st, 2020, Vivet Therapeutics announced today that both the Food and Drug Administration
Vivet Therapeutics Announces 2 Abstracts Presentation At 2020 ASGCT Annual Meeting

Vivet Therapeutics, a privately held gene therapy biotech company
Happy to share the March 2020 Info Wilson newsletter from ABPWilson!

Vivet is very happy to share the March 2020
Vivet presenting during Advanced Therapies 2020 in London

Vivet Therapeutics’ CEO, Jean-Philippe Combal
Vivet Announces Publication in Nature Communications of VTX-803 Preclinical Data

Vivet Announces Publication in Nature Communications of Preclinical Data from VTX-803
Vivet attending Jefferies 2019 London Healthcare Conference

Jean-Philippe Combal, Vivet Therapeutics’ CEO, and Thomas Daniel, BD Director, will be hosting 1×1 meetings during
Vivet Presenting at 2019 World Orphan Drug Congress Europe in Barcelona

Vivet Therapeutics CEO, Jean-Philippe Combal
VIVET THERAPEUTICS PRESENTING DURING AASLD – THE LIVER MEETING 2019 IN BOSTON
Dr. Gloria González-Aseguinolaza, Vivet Therapeutics CSO
Vivet Therapeutics Presenting New Results During 2019 ESGCT Annual Congress in Barcelona

Dr. Gloria González-Aseguinolaza, Vivet Therapeutics CSO & Head of the Gene Therapy

