News
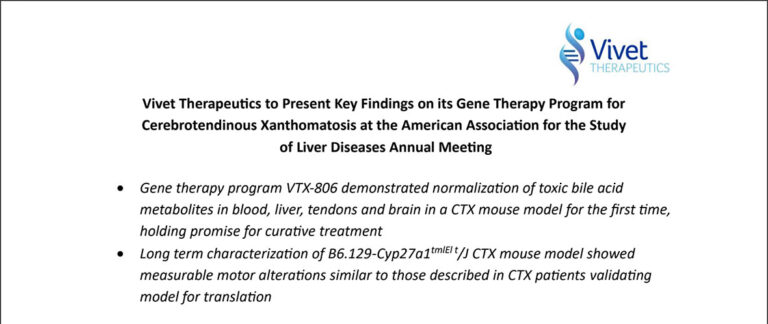
Vivet Therapeutics to Present Key Findings on its Gene Therapy Program forCerebrotendinous Xanthomatosis at the American Association for the Study of Liver Diseases Annual Meeting
Paris, France, November 14, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotechcompany developing novel and long-lasting gene therapies for rare inherited liver metabolicdisorders, today

💗Pink October💗
In collaboration with @Courir pour Elles, @Vivet_tx participated in an 8km walk/run to raise #BreastCancer Awareness and Prevention. Join #TeamVivet in the cause as we

Vivet Therapeutics Presents Three Posters on Cerebrotendinous Xanthomatosis Program and Novel AAV Gene Delivery Platform at European Society of Gene and Cell Therapy Annual Congress 2024
Paris, France, October 22, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic

Leukodystrophy Awareness Month
📢 Leukodystrophy Awareness Month takes place throughout the whole month of September and is a time to raise awareness about these devastating rare conditions! Together
The @European Commission has granted Orphan Drug Designation (#ODD) to our gene therapy product, VTX-806, for the treatment of cerebrotendinous xanthomatosis (#CTX), a rare #neurodegenerativedisease in patients.
European Commission Grants Orphan Drug Designation for Vivet Therapeutics Gene Therapy Product for the Treatment of Cerebrotendinous Xanthomatosis (CTX) • Pre-clinical data of VTX-806 in

Dr Gloria Gonzalez-Aseguinolaza, Chief Scientific Officer of Vivet Therapeutics Receives the Rosalind Franklin Society Special Award in Science
Paris, France, July 16, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic
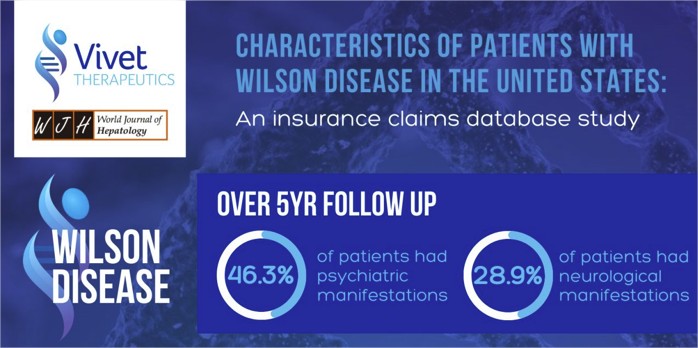
Characteristics of patients with Wilson Disease in the United States: An insurance claims database study
We are delighted to share our findings in a peer reviewed paper published in the World Journal of Hepatology, titled ‘Characteristics of patients with Wilson

Vivet Therapeutics presents interim data on its Phase 1/2 GATEWAY trial for the Treatment of Wilson Disease at EASL Congress 2024
Vivet Therapeutics Presents Interim Data from its Phase 1/2 GATEWAY Trial for the Treatment of Wilson Disease at EASL Congress 2024 VTX-801 increased ceruloplasmin ferroxidase
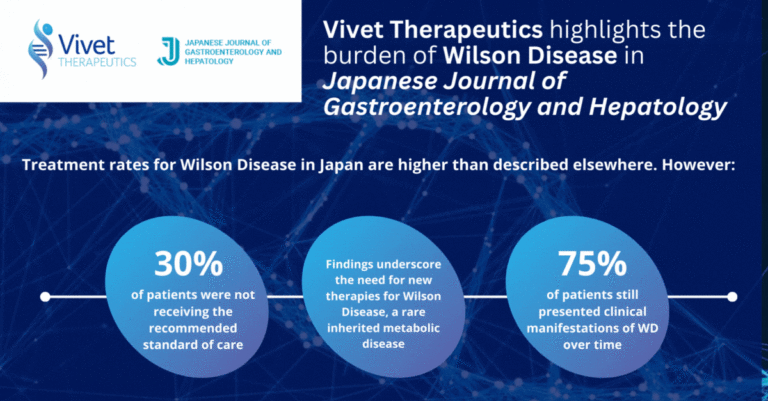
Study on Treatment of Wilson Disease in Japan Published in Japanese Journal of Gastroenterology and Hepatology
Paris, France, May 30, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic
Vivet Therapeutics Doses First Patient in Cohort 2 in Phase 1/2 GATEWAY Clinical Trial for Treatment of Wilson Disease
Vivet Therapeutics Doses First Patient in Cohort 2 in Phase 1/2 GATEWAY Clinical Trial for Treatment of Wilson Disease

Rare Disease Day – February 29
To mark #RareDiseaseDay on 29 February, our CEO Jean-Philippe Combal, spoke to pharmaphorum

Vivet Therapeutics receives EUR 4.9 million to advance development of a gene therapy
PRESS RELEASE Vivet Therapeutics receives EUR 4.9 million to advance development of a gene therapy for the treatment of cerebrotendinous xanthomatosis

Vivet Therapeutics Announces Presentations at Upcoming European Society of Gene and Cell Therapy (ESGCT) 2023 Annual Congress
Paris, France, October 23, 2023 (GLOBE NEWSWIRE) – Vivet Therapeutics (“Vivet”), a clinical- stage biotechnology company

Vivet Therapeutics awarded with the Prix Galien MedStartup in New York
Vivet Therapeutics is very pleased and proud to have won the Prix Galien MedStartup Award for “Best Collaboration For the Developing Or Underserved Populations Worldwide”.

GATEWAY clinical trial for Wilson Disease has now its website
Wilson Disease is a rare, progressive genetic disorder that causes excess copper to be stored in the body.

VTX‐801 Receives U.S. FDA Fast Track Designation for the Treatment of Wilson Disease
VTX-801 Receives U.S. FDA Fast Track Designation for the Treatment of Wilson Disease

Gene Therapy aims to be a one-time treatment that may stop or slow the progression of Wilson disease
Learn how gene therapy aims to target the cause of Wilson disease by delivering a working ATP7B gene into cells. ClinicalTrials are now open for this investigational therapy.

Congratulations to all Women in Gene Therapy and to Dr. Gloria González-Aseguinolaza our CSO @Vivet Therapeutics!
Congratulations to all Women in Gene Therapy and to Dr. Gloria González-Aseguinolaza our CSO @Vivet Therapeutics!

Mirum Pharmaceuticals and Vivet Therapeutics Enter into Exclusive Worldwide Option and License Agreement for Vivet’s PFIC Gene Therapy Programs
Mirum Pharmaceuticals and Vivet Therapeutics Enter into Exclusive Worldwide Option and License Agreement for Vivet’s Gene Therapy Programs Targeting Progressive Familial Intrahepatic Cholestasis

Vivet and Pfizer Inc. Announce FDA Authorization to Proceed with GATEWAY, the Phase 1/2 Study for VTX-801, Vivet’s Investigational Gene Therapy for WD
Vivet Therapeutics and Pfizer Inc. Announce FDA Authorization to Proceed with GATEWAY, the Phase 1/2 Study for VTX-801, Vivet’s Investigational Gene Therapy for Wilson Disease

