Paris, France, May 30, 2024 – Vivet Therapeutics (“Vivet”), a clinical stage biotech company developing novel and long-lasting gene therapies for rare inherited liver metabolic disorders, today announces the publication of a peer reviewed paper titled ‘Treatment of Wilson Disease in Japan: An Insurance Claims Database Study’ in the Japanese Journal of Gastroenterology and Hepatology. Wilson disease (WD) is a hereditary, progressive, severely debilitating disease principally affecting the liver and the central nervous system1. The disease is caused by a defect of copper handling due to mutations in the ATP7B gene, localised on chromosome 13, and encoding the transport protein ATP7B, a copper-transporting ATPase2,3. Loss-of function mutations in this gene lead to failure of excess copper elimination into the bile and tissue accumulation, mainly in the liver and brain, where it causes degenerative injury4,5 and can lead to premature death. The worldwide prevalence has been estimated is 2 to 3.3 cases per 100,0006 with no cure available.
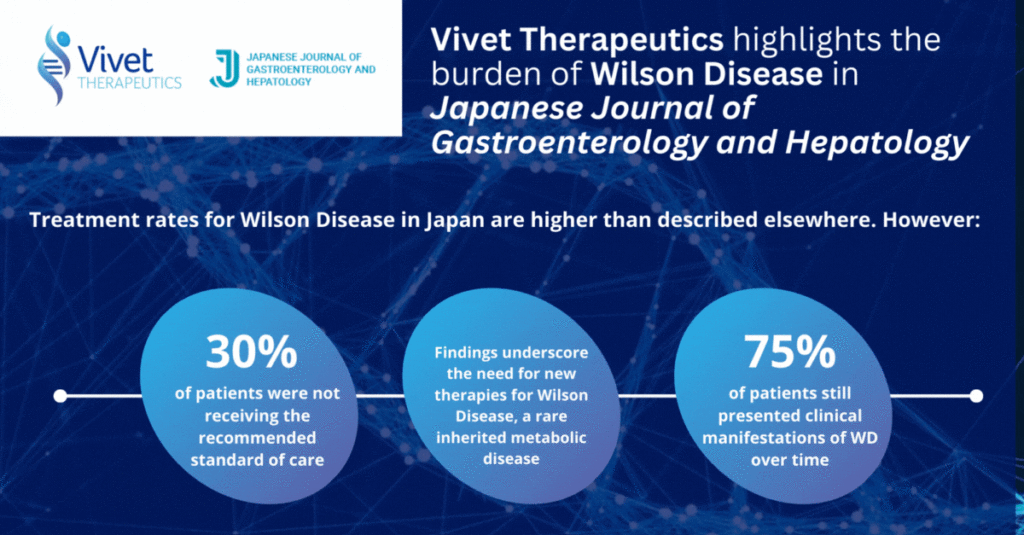
The objective of the study was to evaluate the characteristics and treatment of patients with WD in Japan using the Medical Data Vision (MDV) database. The treatment rates for WD in Japan are higher than other countries in the world, however 30% of patients identified with WD were not receiving the recommended standard of care (SOC) treatment, although coding issues may potentially overestimate such number and 75% of patients still presented clinical manifestations of WD over time. These findings underline the need for a more effective treatment paradigm for WD.
Jean-Philippe Combal, Co-Founder & Chief Executive Officer at Vivet Therapeutics, said: “The study underscores the unmet medical need for new therapies for Wilson Disease. Vivet’s lead program, VTX-801, is currently undergoing clinical evaluation and is a potential disease-modifying treatment for patients with WD.”
Summary of study statistics
•1,952 patients with WD identified, of which 1,131 (57.9%) were analysed
•Estimated prevalence of WD in 2021 was 5.96/100,000
•Mean age of the analysis population was 55.4± 25.2 years
•In 2021, the index date, 850 patients (75.2%) presented clinical manifestations potentially associated with WD
•Following the index date, 830 patients (73.4%) were prescribed a WD-specific treatment
•Clinical manifestations persisted after the index date (82.1% at Year 1 and 75.6% at Year 5), whether patients were prescribed with a WD-specific treatment or not
•During the post-index period,106 patients (9.4%) died in hospital with a mean age of 68.8 years
Further details on the GATEWAY trial can be found on Clinical Trials, under identifier NCT04537377.
Citation:
Citation: Daniel-Robin T. Treatment of Wilson Disease in Japan: An Insurance Claims Database Study. J Gastro Hepato. 2023; V10(3): 1-7
-Ends-
1Wilson S. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver. Brain. 1912; 34: 295-507.
2Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993; 5(4): 327-37.
3Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015; 14(1): 103-13.
4Czlonkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Primers. 2018; 4(1): 21.
5Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann Transl Med. 2021; 9(8): 732.
6Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The Prevalence of Wilson’s Disease: An Update. Hepatology. 2020; 71(2): 722-32.
For further information, please contact:
Optimum Strategic Communications
Mary Clark, Zoe Bolt, Vareen Outhonesack Tel: +44 (0) 20 3882 9621 Email: vivet@optimumcomms.com